Paint, Ink and Coating
Ethyl Acetate Formula, Structure and Properties
Jan 06, 2021
Paint, Ink and Coating
Jan 06, 2021
Ethyl acetate commonly called ethyl ethanoate, is a significant chemical compound extremely utilized as a dissolvable in numerous reactions of chemicals. Ethyl Acetate derivation is extracted from the immediate esterification of ethyl liquor with acidic acid, a method that includes blending acidic acid in with an abundance of ethyl alcohol and adding a modest quantity of sulphuric acid.
The chemical formula of ethyl acetate is CH3COOCH2CH3 and C4H8O2 is its condensed formula. Ethyl acetate or ethyl ethanoate is commonly abbreviated as EtOAc. 88.106 g mL-1 is the molar mass of ethyl acetate. Ethyl acetate is an ester resulting from the acetic acid substitution of the hydroxyl group with an ethoxy group.
Several methods for preparing ethyl acetate exist. The principal strategy is the response between ethanol, acidic acid, and sulfuric acid, at 60-70 °C to facilitate the esterification of carboxylic acid to ester.
CH3COOH + CH3CH2OH → CH3COOC2H5 + H2O
This esterification reaction is a reversible reaction, so an abundance of reagents can be added to increase the yield.
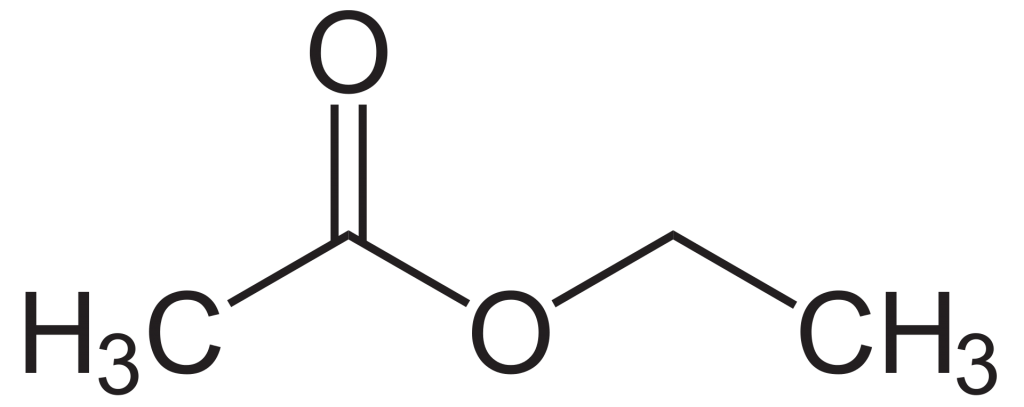
Structure of Ethyl Acetate
Ball and Stick Model of Ethyl Acetate
In general, ethyl acetate is not extensively present. However, since it is highly flammable, it can be used in wine and other alcoholic drinks. A minimal quantity of acetic acid that responds with ethanol to shape ethyl acetic acid derivation is added to these refreshments and alcohols, which is answerable for the flavor of certain old wines. It is additionally utilized as an organic solvent in cellulose, paint, elastic
Ethyl acetate is produced commercially through a distillation method from the fast vinegar method of 10 percent acetic acid and high wines of 50 percent alcohol. First, a ternary combination of ethyl acetate, ethyl alcohol distills, and water. Ethyl acetic acid derivation is crystalline and has a sweet ester of acidic acid and ethanol.
The melting point and the boiling points are -83.6 °C and 77.06 °C, and the density is 0.894 g mL-1. Ethyl acetic acid derivation isn’t dissolvable in water, yet it is dissolvable in the majority of natural solvents, for example, toluene, benzene, and chloroform.
Ethyl Acetate and different esters can be hydrolyzed to the related carboxylic acid and ethanol, and even in the example of ethyl acetate, acids or bases are used as catalysts, and substances to promote the reaction of acetic acid and ethanol are created after a two-stage component.
Transesterification is another significant ethyl acetate reaction where the ester methoxy group can be replaced by an alcohol R- group.
We're committed to your privacy. Tradeasia uses the information you provide to us to contact you about our relevant content, products, and services. For more information, check out our privacy policy.
Leave a Comment